INTROCAN SAFETY 3 22G X 25MM (1") CLOSED SAFETY IV CATHETER (BLUE) 4251128-03 - Box of 50
INTROCAN SAFETY 3 22G X 25MM (1") CLOSED SAFETY IV CATHETER (BLUE) 4251128-03 - Box of 50
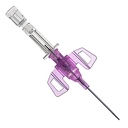
B Braun Introcan Safety 3 Closed IV Catheter 22g x 25mm (1") in Blue
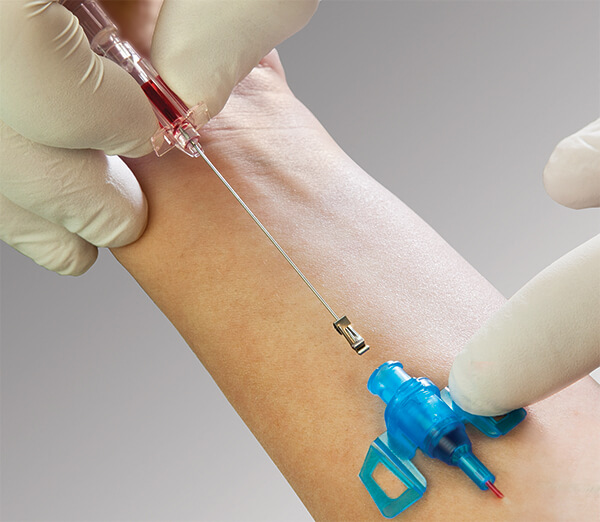
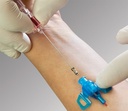
Introcan Safety 3 is a peripheral IV catheter with a passive fully automatic needlestick protection and multi-access blood control septum that helps to protect clinicians and patients from blood exposure. It is a single-use device to generate intravascular and tissue access to sample blood, monitor blood pressure, or administer fluids and blood intravascularly.
Introcan Safety 3 is also indicated for subcutaneous infusion therapies and 300 psi power injector applications (18G – 24G). The device can be used for all patients for which infusion therapy is prescribed. No gender or age-related limitations. Introcan Safety 3 can be used for adults, paediatrics and neonates.
Protecting Healthcare workers against needle-stick injuries and blood exposure.
Enhancing patient care by providing better fixation to help prevent mechanical phlebitis.
Advantages:
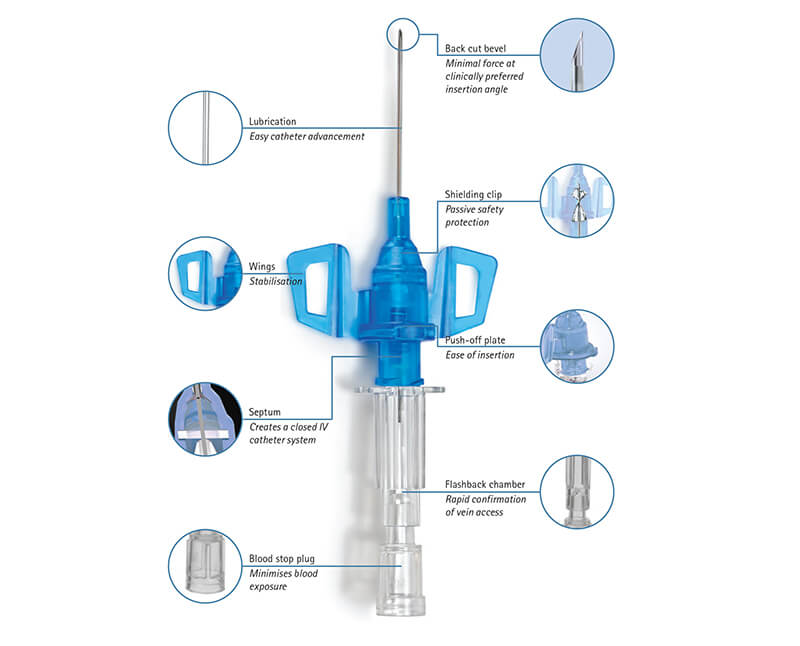
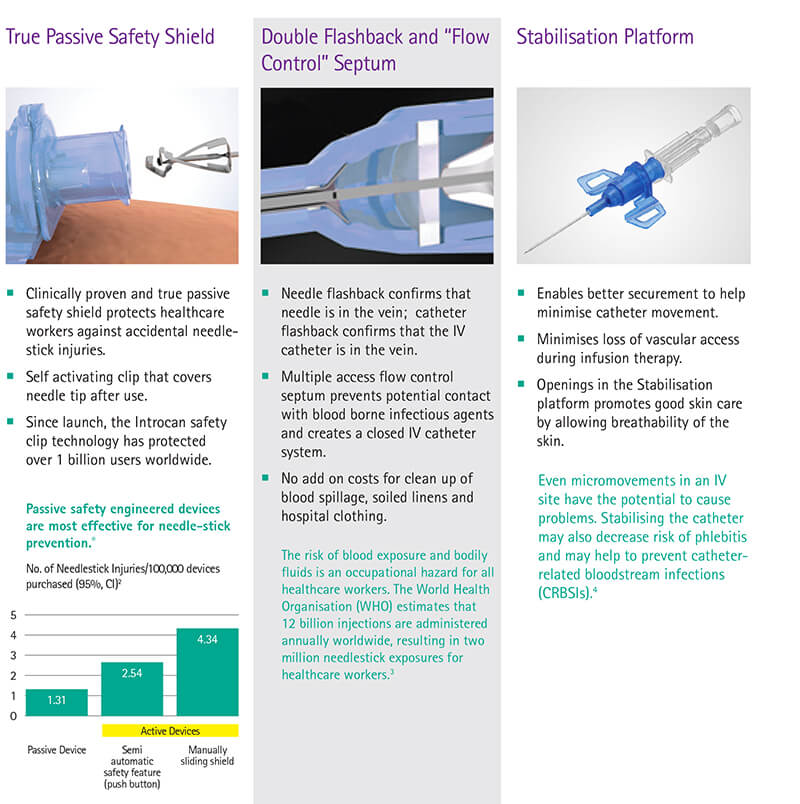
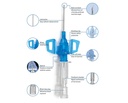
• Passive Safety Shield – A passive fully automatic protection helps eliminate needlestick injuries and related infections. It deploys automatically, cannot be bypassed and requires no user activation
• Multi-access blood control septum – helps to prevent blood exposure during catheter insertion and while disconnecting a device from the catheter hub
• Integrated Stabilization platform – sophisticated catheter securement helps to reduce catheter movement and related complications
• Double Flashback Technology - Confirms that both needle and catheter capillary are inside the vessel. The first needle flash confirms needle is in the vein, second catheter flash confirms catheter is in the vein
• Universal Back Cut Bevel – Allows for a wide choice of insertion angles and is designed for minimal puncture trauma
• Radiopaque Stripes - For good visibility of the catheter capillary under X-Ray
• Power Injectable – Pressure rated for contrast media application at 300 psi (18G – 24G)
• IV catheter material available in PUR and FEP – PUR for a softer and more comfortable in-dwelling performance, FEP as alternative firmer material e.g. for arterial access
• Not made with DEHP, Latex/Natural Rubber, PVC